 Scientists published on the National Institute of Health website reported on three-dozen clinical trials underway, testing the anti-malarial drug Hydroxychloroquine — HQC — to treat cancer.
Scientists published on the National Institute of Health website reported on three-dozen clinical trials underway, testing the anti-malarial drug Hydroxychloroquine — HQC — to treat cancer.
In fact, HCQ is being tested on brain tumors, breast cancer, pancreatic cancer, but also diabetes and arthritis, as well as Lupus and other maladies where it was already known to be an effective treatment. It’s also been found safe enough to be used by pregnant women.
And what’s even more eye-opening, never mind getting a prescription for this cheap generic drug, the active ingredient, otherwise known as Quinine, what’s in tonic water, you can brew at home by steeping a grapefruit peel!
The revelations about the wonder drug came as Remdesivir, the fast-tracked pharmaceutical backed by establishment firm Gilead, were shown to have flopped in a study of 237 patients in China, in draft test results accidentally posted by the World Health Organization, according to a Financial Times report that moved the market and medical news site STAT, which got the WHO screenshot.
When U.S. President Donald J. Trump announced, at the start of the crisis two months ago, that he had a “good feeling” and was “optimistic” that HCQ would prove a powerful match for the Corona Virus, POTUS is being proved right, once again.
Putting his hunch on the line for his own health, on Monday, the president announced at a roundtable at the White House, that a doctor had recommended he take HCQ, along with a zinc supplement, and he’d been doing so, with “zero symptoms” or side effects.
“I’ve taken it about a week and a half now, and I’m still here. I get a lot of tremendously positive news on the hydroxy, and you know the expression I use, ‘What do you have to lose?’” the president said.
b91f159714acd1bcfeeb2676e91845b5e062In a research paper published by scientists on the NIH website in November, 2017, a full list of ongoing studies about HCQ and its original version, CQ, Chloroquine, were compiled where the “well-known” anti-malarial drugs were being used as the treatment or the companion treatment for cancer.
The paper was written by Ciska Verbaanderd, PhD, researcher at Belgium’s University of Leuven in collaboration with the Anticancer Fund, and Gauthier Bouche, MG, MPH, Director of Clinical Research at the Anticancer Fund, where he specializes in drug re-purposing studies.
Their paper references 194 scientific clinical studies.
Just in the Abstract of the study, the punchline is right there:
“Scientific evidence also supports the use of CQ and HCQ in the treatment of cancer.”
Abstract
Chloroquine (CQ) and hydroxychloroquine (HCQ) are well-known 4-aminoquinoline antimalarial agents. Scientific evidence also supports the use of CQ and HCQ in the treatment of cancer. Overall, preclinical studies support CQ and HCQ use in anti-cancer therapy, especially in combination with conventional anti-cancer treatments since they are able to sensitise tumour cells to a variety of drugs, potentiating the therapeutic activity. Thus far, clinical results are mostly in favour of the repurposing of CQ. However, over 30 clinical studies are still evaluating the activity of both CQ and HCQ in different cancer types and in combination with various standard treatments. Interestingly, CQ and HCQ exert effects both on cancer cells and on the tumour microenvironment. In addition to inhibition of the autophagic flux, which is the most studied anti-cancer effect of CQ and HCQ, these drugs affect the Toll-like receptor 9, p53 and CXCR4-CXCL12 pathway in cancer cells. In the tumour stroma, CQ was shown to affect the tumour vasculature, cancer-associated fibroblasts and the immune system. The evidence reviewed in this paper indicates that both CQ and HCQ deserve further clinical investigations in several cancer types. Special attention about the drug (CQ versus HCQ), the dose and the schedule of administration should be taken in the design of new trials.
After pages of summaries of tests on rats, the report compiles five pages of clinical tests underway where HCQ and CQ are showing anti-cancer properties.
HCQ-CQ-tests-cancerTests with CQ were completed against Glioma and brain metastases, with the first going back to 1998, on 38 patients and against Multiple myeloma, with 11 patients.
HCQ was tested on solid cancers, with 27 patients, again on 40 patients, on 27 and again on 25; against Glioblastoma, on 92 patients; on small-cell lung cancer, 27 patients; against Multiple myeloma, on 25 patients; against Pancreatic cancer, on 35 patients, and on 20 patients; against Sarcoma, on 10 patients.
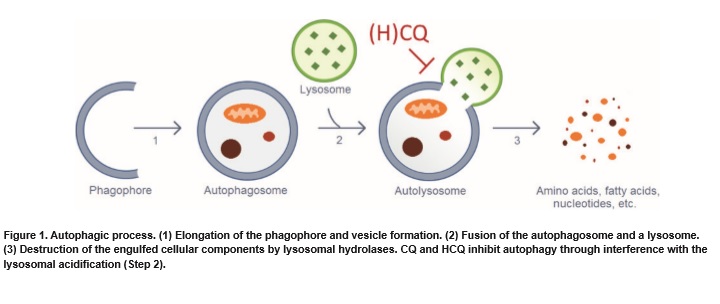
As to how it works, the researchers theorized that they both tackle the cancer cells and change the environment around the cancer to help other treatments, including chemotherapy, and the body, itself, to do the job.
Multiple hypotheses have been proposed on how CQ and HCQ exert their anti-cancer activity. Most studies reported the direct action of these drugs on cancer cells, but more recent studies have also mentioned important effects of CQ and HCQ on the tumour microenvironment. Based on preclinical studies, it is safe to say that CQ and HCQ have multiple mechanisms of action that might complement each other.
The conclusion was only that the drugs do have impact and are interesting study, and that the side-effects were limited, and much more limited than the most high-impact cancer treatments, such as radiation and chemotherapy.
More than 30 clinical trials are currently ongoing (Feb 2017). The results of these trials may indicate which tumour types are most sensitive to CQ and HCQ treatment, and which combination therapies can be beneficial… CQ and HCQ have been studied in multiple preclinical cancer models and have demonstrated activity on several cancer-supporting pathways and in combination with a broad range of other therapies. Our review has highlighted the interesting multi-faceted actions of CQ and HCQ against cancer, making these drugs attractive for this complex disease.
Their work only goes into the impact on cancers, but the gauntlet has been picked up by many others, building on their research. Google Scholar, which tracks scientific papers, found their work referenced by 53 other research projects.
HIV, Q fever, Whipple’s disease, fungal infections, systemic lupus erythematosus, antiphospholipid antibody syndrome, rheumatoid arthritis, Sjögren’s syndrome
neurosarcoidosis, chronic lymphocytic inflammation, progressive multiple sclerosis. safe even during pregnancy
A grocery list of maladies treated by HCQ and CQ is included in the abstract of ‘Current and Future Use of Chloroquine and Hydroxychloroquine in Infectious, Immune, Neoplastic, and Neurological Diseases: A Mini-Review’, by Dr. Domenico Plantone, of the Nuerology unit of the Multiple Sclerosis Center, at the Regina Elena National Cancer Institute, and Tatiana Koudriavtseva, MD, PhD, of IFO – Istituti Fisioterapici Ospitalieri, both in Rome.
Chloroquine and hydroxychloroquine, with an original indication to prevent or cure malaria, have been successfully used to treat several infectious (HIV, Q fever, Whipple’s disease, fungal infections), rheumatological (systemic lupus erythematosus, antiphospholipid antibody syndrome, rheumatoid arthritis, Sjögren’s syndrome), and other immunological diseases. Indeed, they have anti-inflammatory, immunomodulating, anti-infective, antithrombotic, and metabolic effects. Among the biological effects of chloroquine and hydroxychloroquine, it is important to highlight their antitumoral properties, likely due to their strong antiproliferative, antimutagenic, and inhibiting autophagy capacities. These effects make these drugs a possible option in the treatment of several tumors in association with radiotherapy and chemotherapy. Finally, the repurposing of chloroquine and hydroxychloroquine is currently being examined for neurological diseases such as neurosarcoidosis, chronic lymphocytic inflammation with pontine perivascular enhancement responsive to corticosteroids, and primary progressive multiple sclerosis. Several ongoing clinical trials have been testing these drugs in non-neoplastic and neoplastic diseases. Moreover, the well-demonstrated good tolerability of chloroquine and hydroxychloroquine make them safe even during pregnancy. Gastrointestinal and cutaneous manifestations are considered not to be serious, while retinal, neuromuscular, and cardiac toxicities are classified as serious adverse events.
In a paper published in January, 2019, in Clinical Rheumatology, the eight scientists describe HCQ’s impact on arthritis:
We describe the first series using hydroxychloroquine as a first-line disease-modifying antirheumatic drug (DMARD) for patients without pre-existing autoimmune disease, who developed arthritis secondary to ICI’s… In our experience, in patients without pre-existing autoimmune disease, hydroxychloroquine is an effective first-line therapy for IA secondary to ICI therapy.
A paper published in April, 2019, in Oncogene, reports on CQ’s impact T-cell acute lymphoblastic leukemia (T-ALL):
Here, we show that the clinically used anti-malarial drug chloroquine (CQ), an inhibitor of lysosomal function and autophagy, decreases T-ALL cell viability and proliferation.
The nine scientists also include a description of what’s at play:
CQ impairs the redox balance, induces ds DNA breaks and activates the DNA damage response. CQ also interferes with intracellular trafficking and processing of oncogenic NOTCH1. Interestingly, we show for the first time that the addition of CQ to γ-secretase inhibition has a synergistic therapeutic effect on T-ALL and reduces the concentration of GSI required to obtain a reduction in cell viability and a block of proliferation. Overall, our results suggest that CQ may be a promising repurposed drug in the treatment of T-ALL, as a single treatment or in combination with GSI, increasing the therapeutic ratio.
Working down the line of serious diseases, just type in HCQ and anything, say, Diabetes, into Google Scholar, and a paper comes up describing the research and its positive impact.
Mercer2012_Article_HydroxychloroquineImprovesInsuThis 2012 research paper describes HCQ as “a common disease modifying therapy for the treatment of rheumatoid arthritis” where prior research showed it may have an effect on patients with diabetic symptoms; lo and behold, these six scientists treated 13 “obese, non-diabetic subjects without systemic inflammatory conditions” with HCQ for six weeks and then tested them without it after another six weeks, and it helped.
Conclusion:
In this small pilot study we found that during HCQ use, obese non-diabetic subjects experienced a significant benefit in insulin sensitivity… This argues for a direct effect of HCQ on insulin metabolism-reduced degradation or enhanced activity at the receptor level, rather than an indirect effect through reduced inflammation.
So, there’s definitely something to it.
Now, the same can’t be said of Gilead’s faux wonder drug, Remdesivir.

On Monday, Gilead upped its donation to the federal government from 607,000 doses to around 940,000, with the implied rationale that more of something mediocre is better than less of something mediocre.
If you understood Gilead as a blue chip pharmaceutical company, “a winner,” it might come as a surprise that Remdesivir was first produced to fight Ebola, but proved ineffective. It was then applied to MERS and SARS, which are related to Corona Virus, and had some positive results in animal testing, but not really in humans, never won the blue ribbon, yet it’s always at the front of the line.
The answer is in that Gilead was initially funded and backed by former Secretary of Defense Donald Rumsfeld and George Schultz, President Ronald Reagan’s Secretary of State, along with the founder of Intel and the former chairman of Sears Roebuck.
This old article starts: “For a small biotech company with no profits, Gilead Science has friends in high places.”
And what did they do with this juggernaut over the years?
“The company’s HIV drugs continue to rake in billions of dollars in sales. Gilead has emerged as the leader in CAR-T cell therapies for treating cancer. It could also soon stake a claim in the massive immunology market if filgotinib wins FDA approval later this year in treating rheumatoid arthritis.”
(As for filgotnib, not approved yet, still in trials, known side effect: testicular toxicity, as in affecting the production of sperm cells.)
The ugly truth that came out last week in the WHO document posted online showed no impact of Remdesivir on patient health or on reducing the Corona Virus in their bodies.
“In response to WHO asking for information and studies to be shared early, a draft document was provided by the authors to WHO and inadvertently posted on the website and taken down as soon as the mistake was noticed,” according to the WHO, for why the data was posted, while Gilead warned of “inappropriate characterisations of the study.”
“Importantly, because this study was terminated early due to low enrollment, it was underpowered to enable statistically meaningful conclusions,” Gilead said. “As such, the study results are inconclusive, though trends in the data suggest a potential benefit for remdesivir, particularly among patients treated early in disease.”
These were 237 patients in China who the drug failed, while the hundreds of patients with “positive outcomes” of HCQ are discounted.
While shares of Gilead dropped 4% on the bad news, the description of the context — the ‘nut ‘graph — is telling from Reuters:
Interest in Gilead’s remdesivir has been high as there are currently no approved treatments or preventive vaccines for COVID-19, and doctors are desperate for anything that might alter the course of the disease that attacks the lungs and can shut down other organs in extremely severe cases.
In fact, the whole article was a positive spin on top of hyperbolic spin, swirling around the vapors of that nothingness, otherwise known as very bad news:
David Katz, chief investment officer at Matrix Asset Advisors, which owns [an undisclosed amount of] Gilead shares, noted that multiple clinical studies remained ongoing for remdesivir and said, “we would not be selling (the stock) into weakness today.”
Gilead is awaiting results of a trial of remdesivir, which previously failed as an Ebola treatment, in 400 patients hospitalized with severe COVID-19, with data expected later this month. A separate trial in China testing the drug in patients with more moderate symptoms last week was also suspended due to a lack of eligible patients.
Brad Loncar, whose Loncar Investments runs The Cancer Immunotherapy ETF, was not ready to draw conclusions from the latest China data. “Just as it was too much of a reach to be optimistic about positive anecdotes we have heard, I think it’s too pessimistic to write it off based on this (incomplete) data.”
Pointedly, the article makes no mention of HCQ.
And as for HCQ, it turns out that the CDC, since 2005, was aware this old anti-malaria drug fought the Corona Virus, while researchers in China, Korea and France had been publishing the same since January when it came to their attention as a potential cure as the plague beset them.
First published in August, 2005, the CDC Special Pathogens Report confirms the Centers for Disease Control knew that CQ could be used to treat the infection and spread of SARS corona virus.
In January of this year, Chinese cell researchers Manli Wang, Ruiyuan Cao, Leike Zhang, Xinglou Yang, recognized CQ as a cheap and a safe drug that was potentially clinically applicable against the 2019-nCoV strain.
In February, guidelines from South Korea and China reported that HCQ and CQ were effective antiviral therapeutic treatments for the virus.
In March, French scientists began an open-label non-randomized clinical trial by Didier Raoult M.D/Ph.D et al, completed in April. The Summary report finds that 100% of patients who received a combination of HCQ and Azithromycin tested negative and were virologically cured within six days of treatment.
The quick and dirty answer to all this is HCQ works and is nearly free because it’s a half-century old generic drug; meanwhile, big companies have bet billions on finding an “alternative” cure they can’t prove, but can protect by patent. The price difference is $0.64 vs. $1,000.00.
They’ve turned the screw of the establishment so hard to protect that turf that even in the heart of Texas, where HCQ was once prescribed “off-label,” meaning doctors could offer it to patients even though it was not approved for all these uses, now you only can get it with correct paperwork.
Dr. Ivette Lozano, who has been in medical practice for over 20 years, was shocked to learn in March that she would be required to share her patient’s diagnosis with the pharmacist before prescriptions for hydroxychloroquine and azithromycin would be dispensed.
“Never before have we had to turn in a diagnosis with a prescription,” Dr. Lozano told The Texan. Lozano has seen about five to six patients per week for coronavirus. “They see a dramatic improvement within six to eight hours,” Lozano said.
The unseemly prelude to all this is that the billionaire owners of Apotek — the Canadian generic drug giants and one of the world’s largest producers of HCQ — were ritually murdered, with their dead bodies posed by their killers to mimic a sculpture in their home.
Why? Because they were charitable folk who, to save lives, would have happily given out their antidote pill for free.

Now, it’s one thing to get a magic pill for free, but the only thing that could Trump that is the real truth.
Forget a magic pill, you can make the antidote at home.
In the kitchen, all you need is a pot on the stove. And a grapefruit peel.
Quinine — as in “tonic with quinine” — can be made from boiling the rinds of a couple ripe grapefruits under a few inches of water, letting it evaporate against a glass-topped pot; simmering that airtight for two hours, then recover all the liquid once it cools. Voila.
Sweeten with honey, take a tablespoon every few hours to bring up the phlegm. Or, pour a cup of it, sweeten liberally, mix with OJ or drink as tea.
(Or, for the gentlemen, mix it with seltzer water, and take it with your favorite gin.)
Swallow some zinc pills, it mainlines the quinine straight into your cells.
Forget the store bought “tonic”, even if it weren’t so fake, you’d need a dozen liters a day to get the effect. But think back to when “gin and tonic” was in its heyday, 1800s India, where the combo dulled the senses, but also fought off malaria.
Quinine is real.
Jentox — aka Jen Shumaker — trained biochemist, former CDC scientist, then classically trained theater actress, and now Certified Natural Health Professional — can walk you through it, above, or read more where she’s referenced in another article, ‘How I discovered the benefits of quinine’. Here’s a recipe to make an essential oil of quinine, though drinking it as a liquid is far more powerful, though this would be therapeutic, especially if you inhale the vapors into the lungs for a bad corona cough.
President Trump — the guy with the “good feeling” who’s “optimistic” about HCQ, and now turns out to be taking it daily — recently said:
“In one year, every treatment that we are now using in the hospitals will be obsolete.”
Because of HCQ?
Here’s to raising a gin and tonic to that!
RELATED ARTICLES:
https://resonant.fr/military-roots-of-wonder-drug-hcq/
https://resonant.fr/in-2005-cdc-knew-anti-malaria-drug-active-against-virus-so-why-the-wait/
http://resonant.fr/big-pharma-donates-millions-of-doses-of-anti-malaria-drug-for-corona-virus/
http://resonant.fr/dr-judy-mikovits-gates-of-hell-bring-prima-fauci-case-against-americas-doctor/



A-mazing, thank you, well done — RS
Wowzers! we knew the Prez had the hunch, and the Prez is always right,,, so, right on!
Carry on, soldiers! Greet work, keep it up!
Lots of FB comments and letters to us on this one:
1. Yes, HCQ from a pill and quinine from a peel or chinchona from bark are not exactly, exactly the same, but close enough, of similar chemical structure based on what’s found in nature, and provide similar positive effect on the body.
– 1b. This stuff kills the virus! Why are you waiting?
2. Forthcoming, we can do a ‘how to’ post on cooking it up at home — lots of eager reader questions on this one.
– 2b. Did it with 3 grapefruit peels in a ceramic pot, came out great, made up a half liter, lasting a week.
– 2c. Mixing it with equal parts, tangerine juice, soda water, tequila. (We finished the gin at the start of the quarantine!)
3. No, don’t know how ‘powerful’ a cup of home brew is, but have asked some experts, we shall see.
– 3b. Strong stuff, for sure, keep it to a shot in your cup — don’t over serve!
– 3c. Yes, we can do another report describing chinchona bark tonic, but here’s a great link courtesy of a poster at Free Republic, which picked up this story: https://www.freerepublic.com/focus/f-chat/3847787/posts
https://pinchandswirl.com/homemade-tonic-water-for-the-ultimate-gin-and-tonic
– 3d. Also, see how HCQ was used forever by the military http://qm.news/military-roots-of-wonder-drug-hcq/
I prefer Vodka with my quinine water………Schwepps please!